Improve the way you manage transparency

The world’s first end-to-end transparency platform
The world’s first end-to-end transparency platform
Consolidate
Assess
Plan
Author
Review & Approve
Submit
Consolidate
Plan
Review & Approve
Assess
Author
Submit

Consolidate
Import and triage all CTMS, spreadsheet and SAS data into a single, consolidated form.
Consolidate
Import and triage all CTMS, spreadsheet and SAS data into a single, consolidated form.

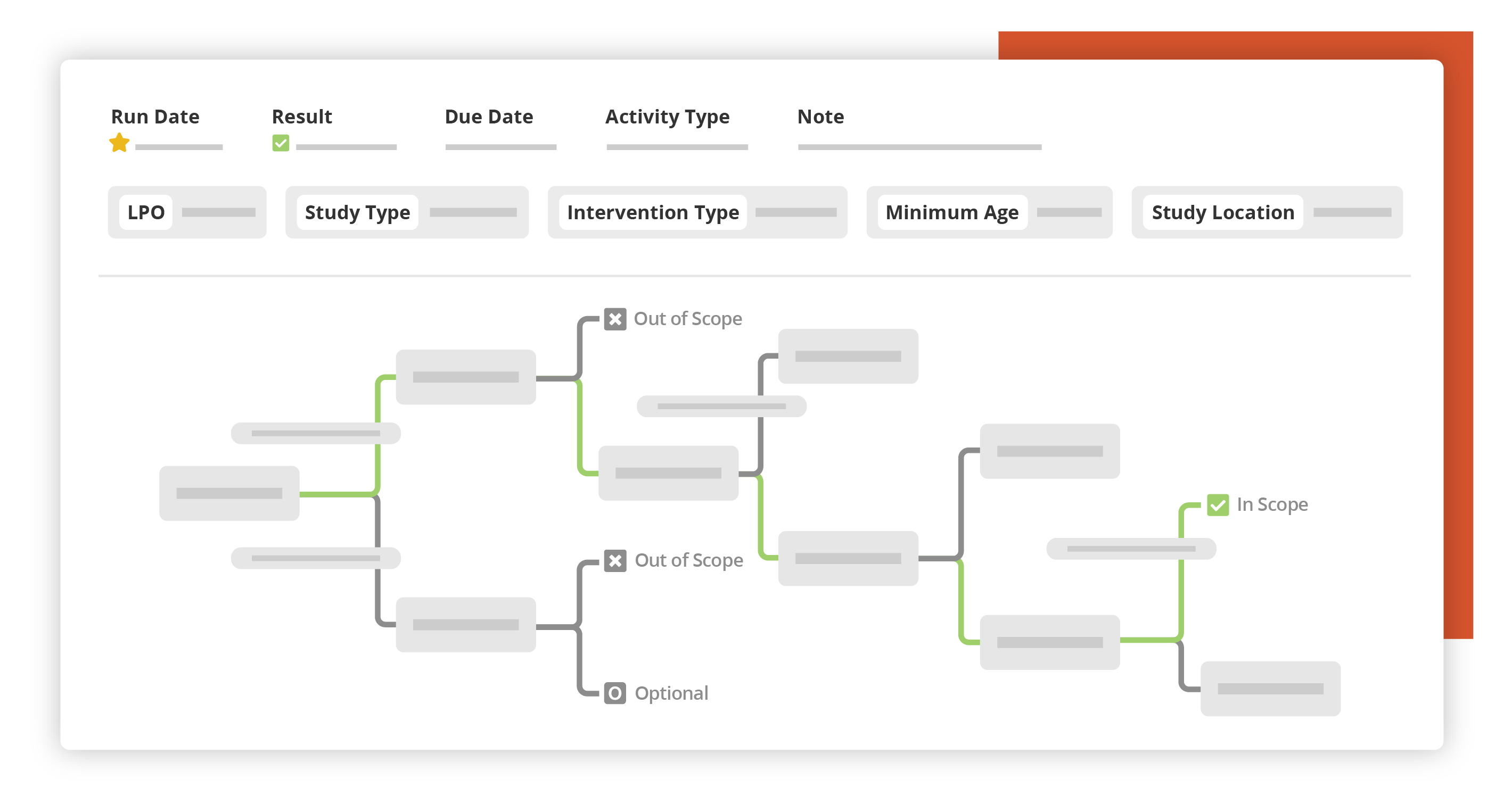
Assess
Automatically assess your portfolio against global and local requirements with our monitored decision trees.
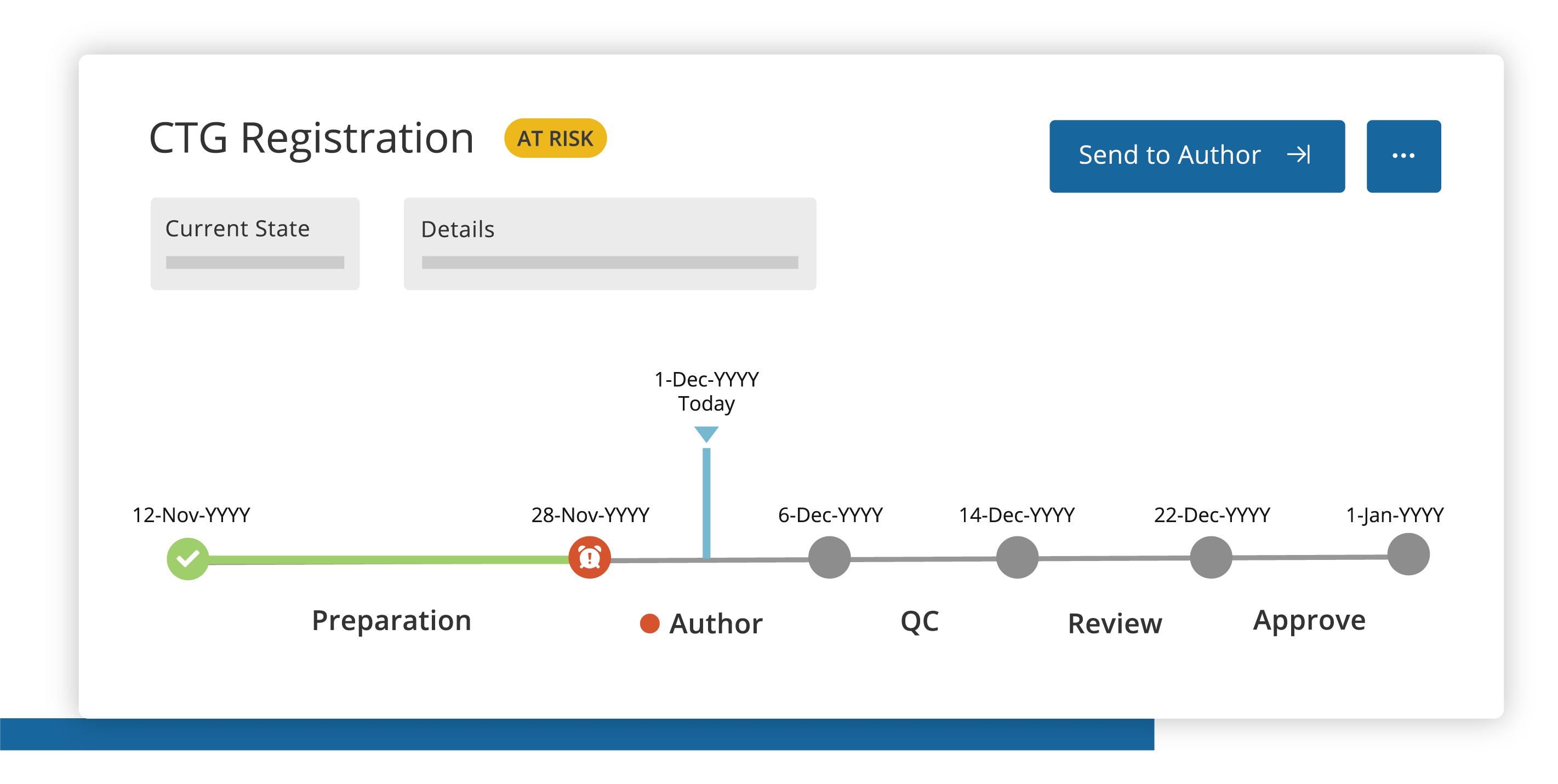
Plan
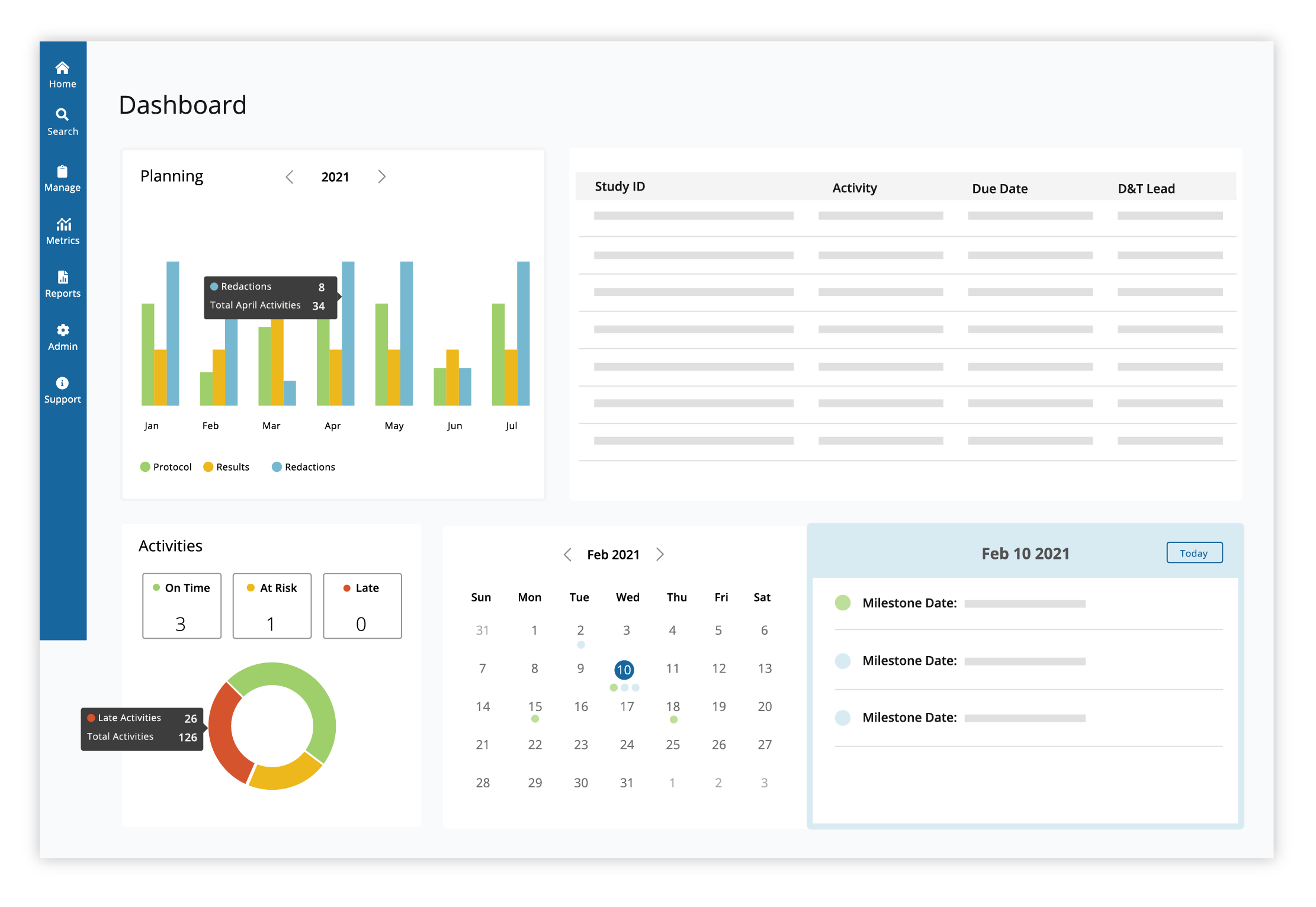
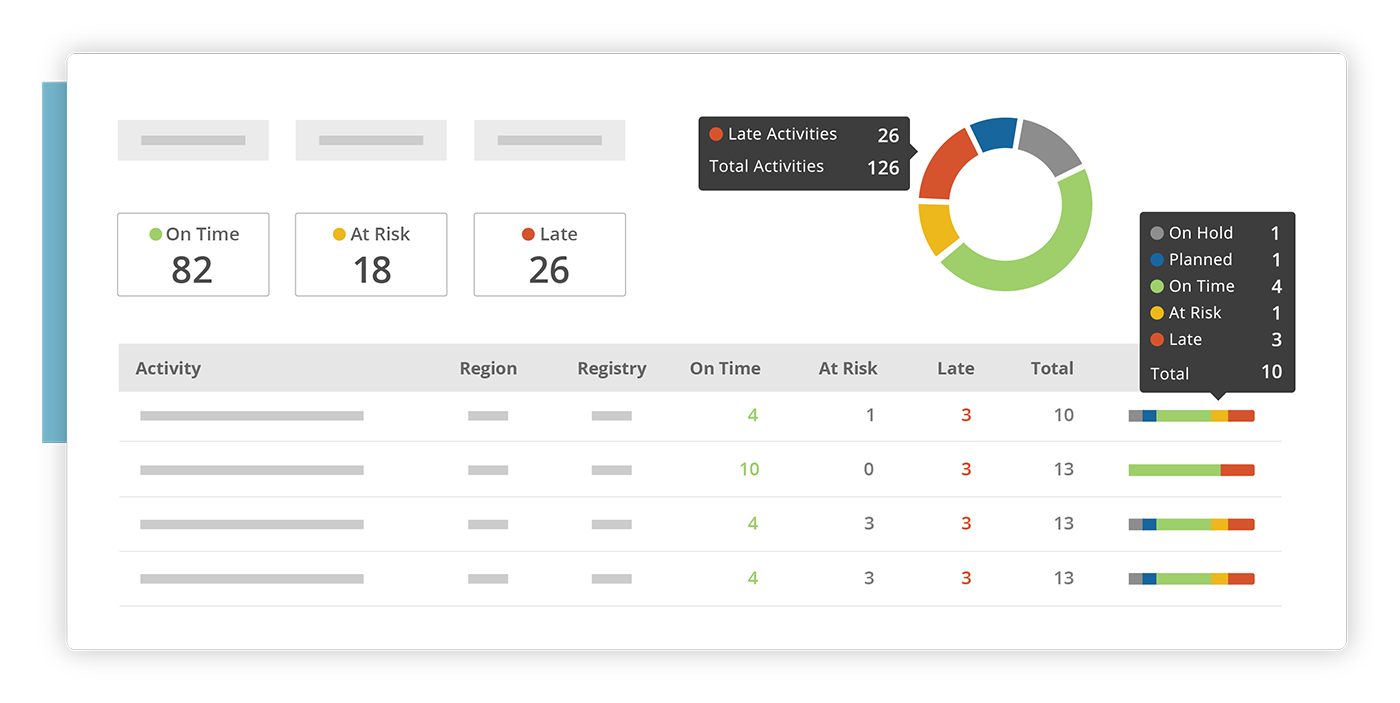
Manage past, current and future transparency activities with intuitive project tracking, dashboards and detailed reports.
Plan
Manage past, current and future transparency activities with intuitive project tracking, dashboards and detailed reports.

Author
Streamline data entry with a single, consolidated form and up to date registry validation.
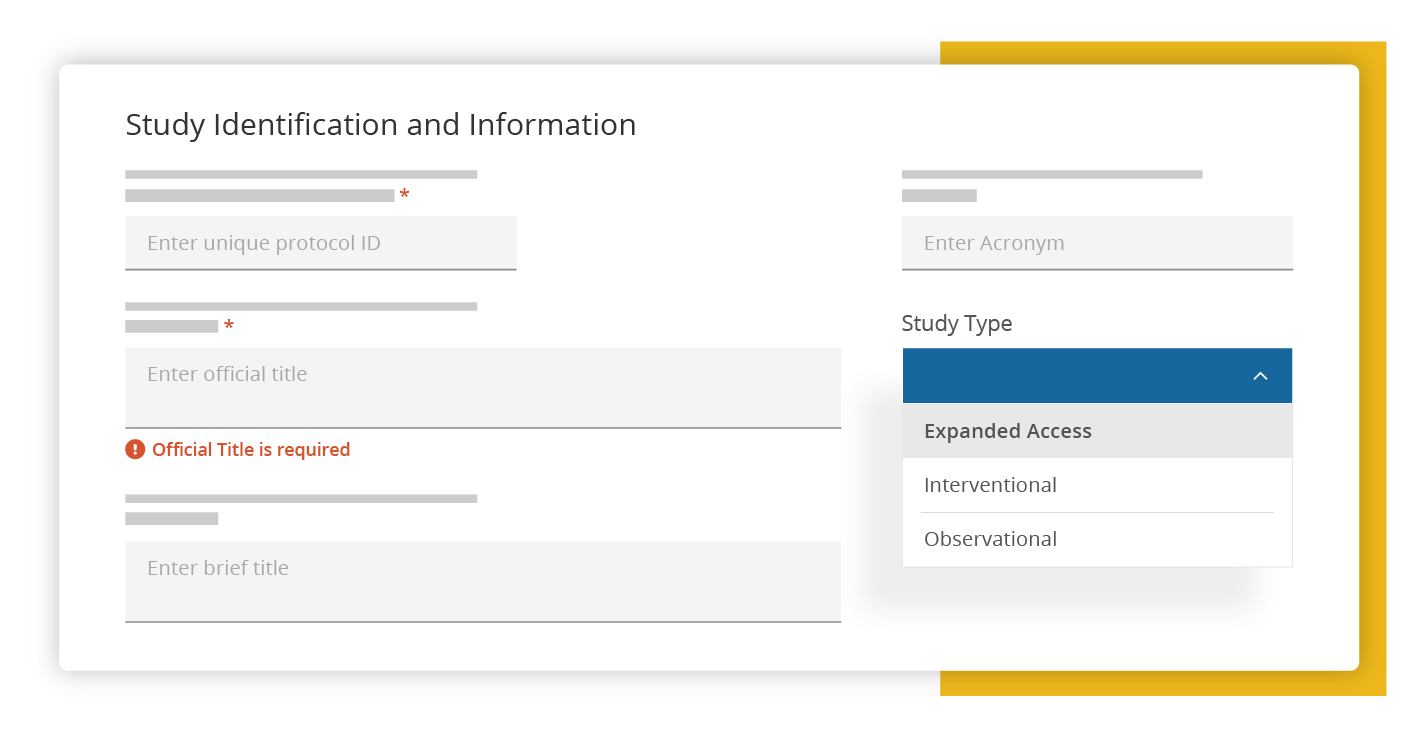
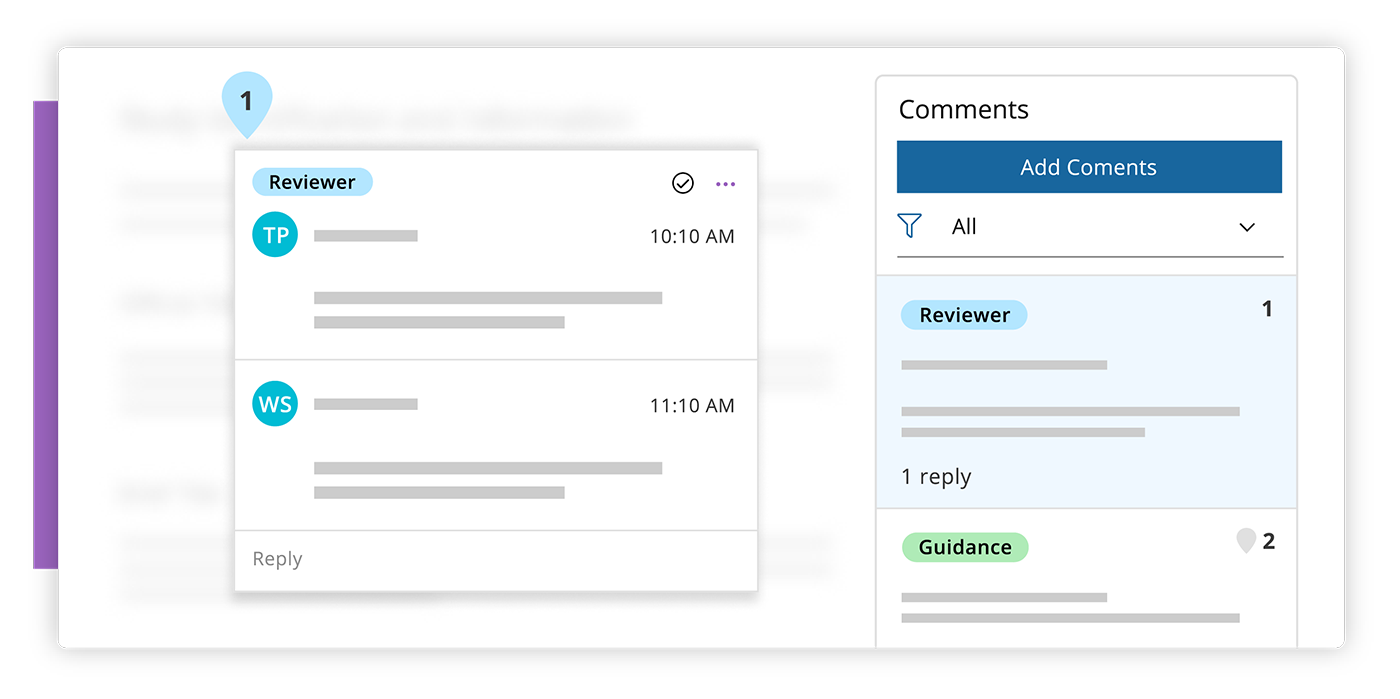
Review & Approve
Easily review and approve data with enhanced commenting capabilities.
Review & Approve
Easily review and approve data with enhanced commenting capabilities.

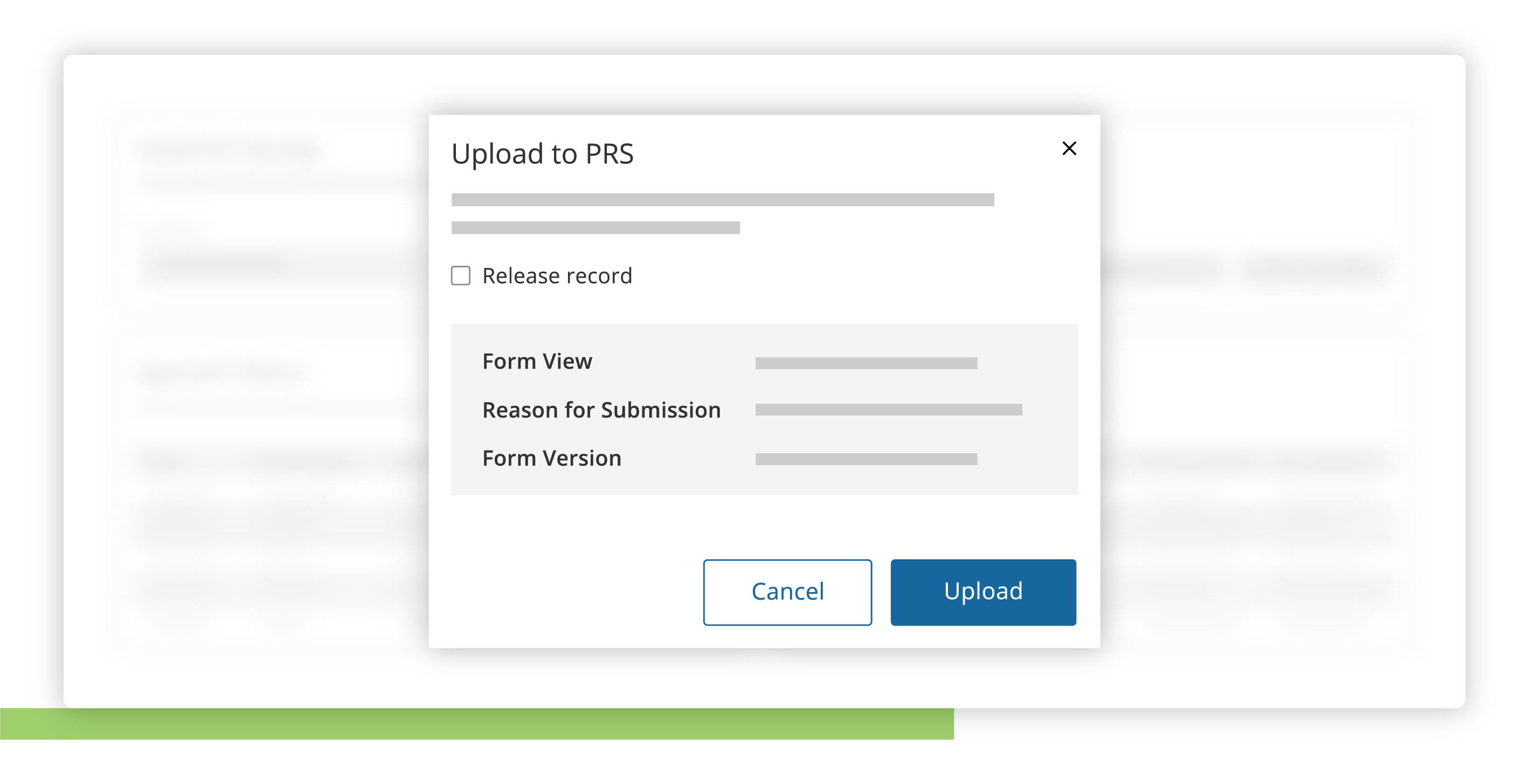
Submit
Seamlessly submit trial data to supported registries.
How we are different
| Prime | Our Competitors | No Solution | ||
|---|---|---|---|---|
| CTMS Integration |  |
 |
||
| Source System Data Override |  |
|||
| SAS Data Import |  |
|||
| Consolidated Data Form |  |
|||
| Global Regulatory Monitoring |  |
 |
||
| Global Activity Tracking |  |
 |
||
| Activity Planning Dashboard |  |
|||
| Up to date Registry Validation |  |
 |
 |
|
| Data Entry Aids |  |
|||
| Workflow Configuration |  |
 |
||
| Email based Review & Approval |  |
 |
||
| Intuitive Review & Approval |  |
|||
| Enhanced Commenting Capabilities |  |
 |
||
| Seamless Submission to CTG |  |
 |
 |
|
| Seamless Submission to EudraCT |  |
 |
||
| CTG PRS Comment Visibility |  |
 |
||
| CTG Release Receipt Retrieval |  |
 |
 |

Want to see a demo?
About Us
Careers
Solutions
Prime
Reach
RadarX
Services
Regulatory Intelligence
EU CTIS Submission Support
Advisory Services
©2024 Xogene LLC
Terms & Conditions
Privacy Policy
Terms & Conditions