Planning a CTA submission to the EU Clinical Trial Information System (CTIS)?
We’ve got you covered.
With the EU Clinical Trial Regulation (CTR) 536/2014 in effect as of January 31, 2022, sponsors are beginning to submit their Clinical Trial Applications (CTA) to EU Clinical Trials Information System (CTIS). We have developed expertise through monitoring of clinical trial regulations, active participation in dedicated focus groups, and direct experience in helping sponsors with pilot submissions.

We can help you get ready and navigate the complex CTR landscape.
Our highly trained and experienced team ensures consistent, accurate disclosure of trial documents. We understand the science, systems, and laws driving the increasing clinical trial disclosure and transparency requirements. We always strive for excellence in quality and compliance.
Using artificial intelligence, machine learning and pattern recognition, we leverage innovative in-house technologies to deliver effective anonymized and redacted clinical trial documents for submissions.
For sponsors taking the important step toward implementation of plain language summary initiatives, Xogene has the critical skills to bridge the gap between medical and plain language terminologies. Our specialists and plain language writers ensure trial information is optimized for accessibility, improves health literacy, and maximizes patient engagement. We further enhance the information for clarity with the use of infographics created by our in-house team of graphic designers.
We can help you get ready and navigate the complex CTR landscape.
Our highly trained and experienced team ensures consistent, accurate disclosure of trial documents. We understand the science, systems, and laws driving the increasing clinical trial disclosure and transparency requirements. We always strive for excellence in quality and compliance.
Using artificial intelligence, machine learning and pattern recognition, we leverage innovative in-house technologies to deliver effective anonymized and redacted clinical trial documents for submissions.
For sponsors taking the important step toward implementation of lay summary initiatives, Xogene has the critical skills to bridge the gap between medical and plain language terminologies. Our specialists and plain language writers ensure trial information is optimized for accessibility, improves health literacy, and maximizes patient engagement. We further enhance the information for clarity with the use of infographics created by our in-house team of graphic designers.

We can get you EU CTR 536/2014 ready
We can get you EU CTR 536/2014 ready
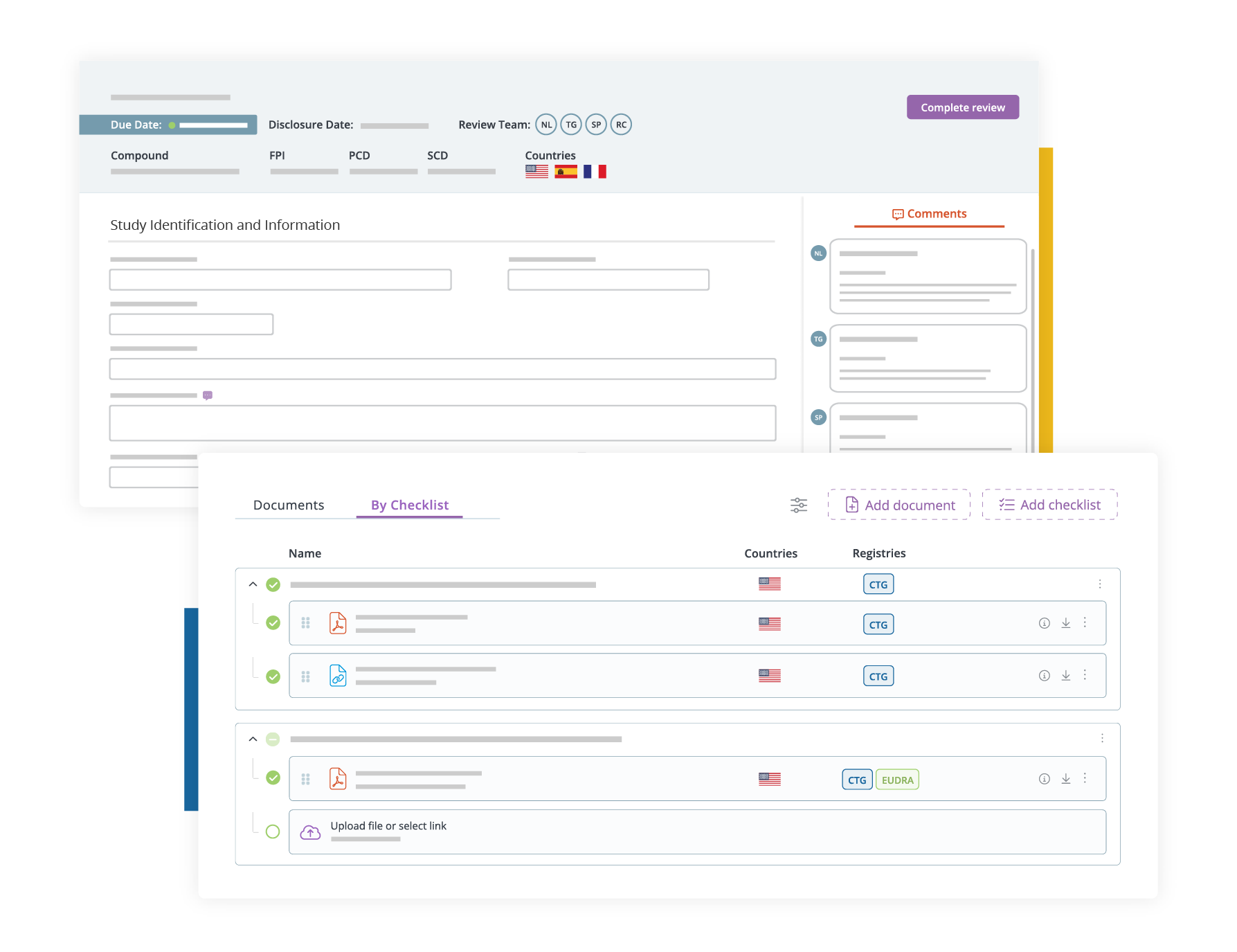
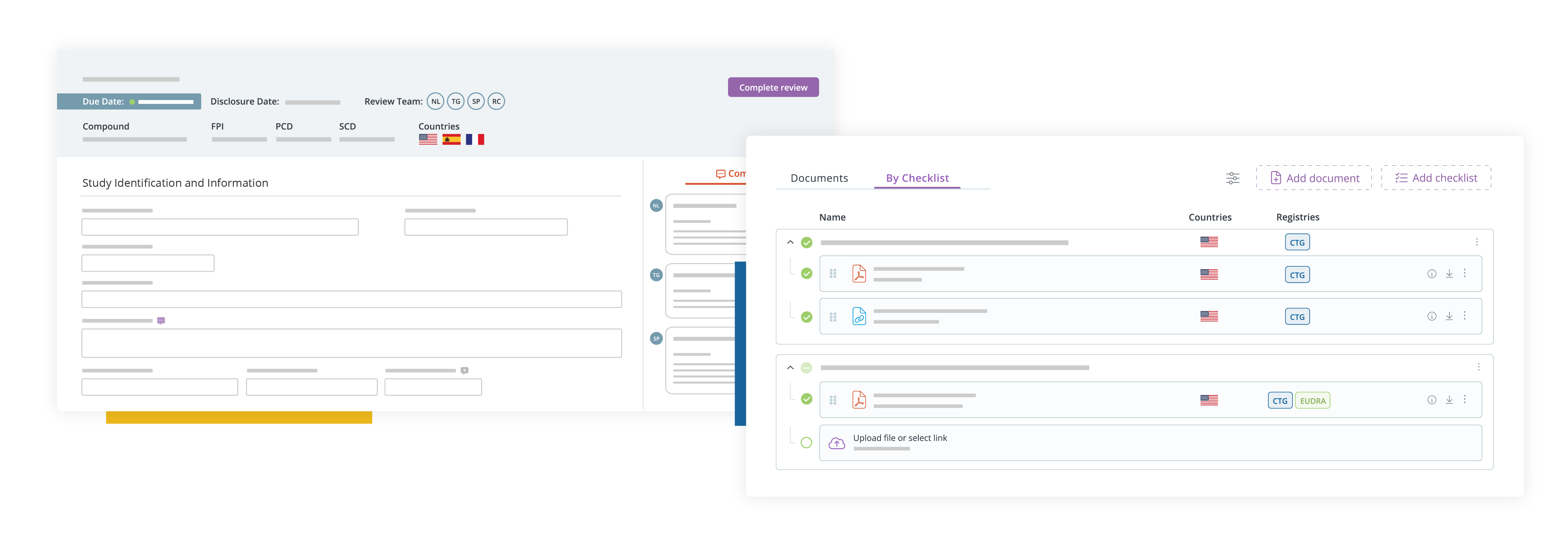
We offer a full range of services to support Clinical Trial Application submission to EU CTIS. More specifically, our solutions include the following:
- Plain Language Protocol Synopsis
- Redaction of Part I and Part II Documents for Initial Submission and Subsequent Modifications
- Preparation of Package with For Publication and Not for Publication Documents
- Translation of Submission Documents
- Support with Preparation of Documents in Response to RFIs
- Intermediate and Final Results Submission
- Plain Language Summaries of Results and Translations
- Submission of De-identified (redacted/anonymized) Clinical Study Report Post-Marketing Authorization
- Document Checklist for Submission Tracking
- Authoring and Review of WHO ICTRP Data Elements
- Translation Checklist
- Educating Teams on EU CTIS Submission Requirements
- Plain Language Protocol Synopsis
- Redaction of Part I and Part II Documents for Initial Submission and Subsequent Modifications
- Preparation of Package with For Publication and Not for Publication Documents
- Translation of Submission Documents
- Support with Preparation of Documents in Response to RFIs
- Intermediate and Final Results Submission
- Plain Language Summaries of Results and Translations
- Submission of De-identified (redacted/anonymized) Clinical Study Report Post-Marketing Authorization
- Document Checklist for Submission Tracking
- Authoring and Review of WHO ICTRP Data Elements
- Translation Checklist
- Educating Teams on EU CTIS Submission Requirements
Got questions?
We're here to help.
Got questions?
We're here to help.
By submitting this form, you agree to Xogene’s Terms of Service and Privacy Policy and also agree to receive emails from Xogene on educational resources, events, and product updates. You can unsubscribe at any time.
About Us
Careers
Solutions
Prime
Reach
RadarX
Services
Regulatory Intelligence
EU CTIS Submission Support
Advisory Services
©2024 Xogene LLC
Terms & Conditions
Privacy Policy
Terms & Conditions