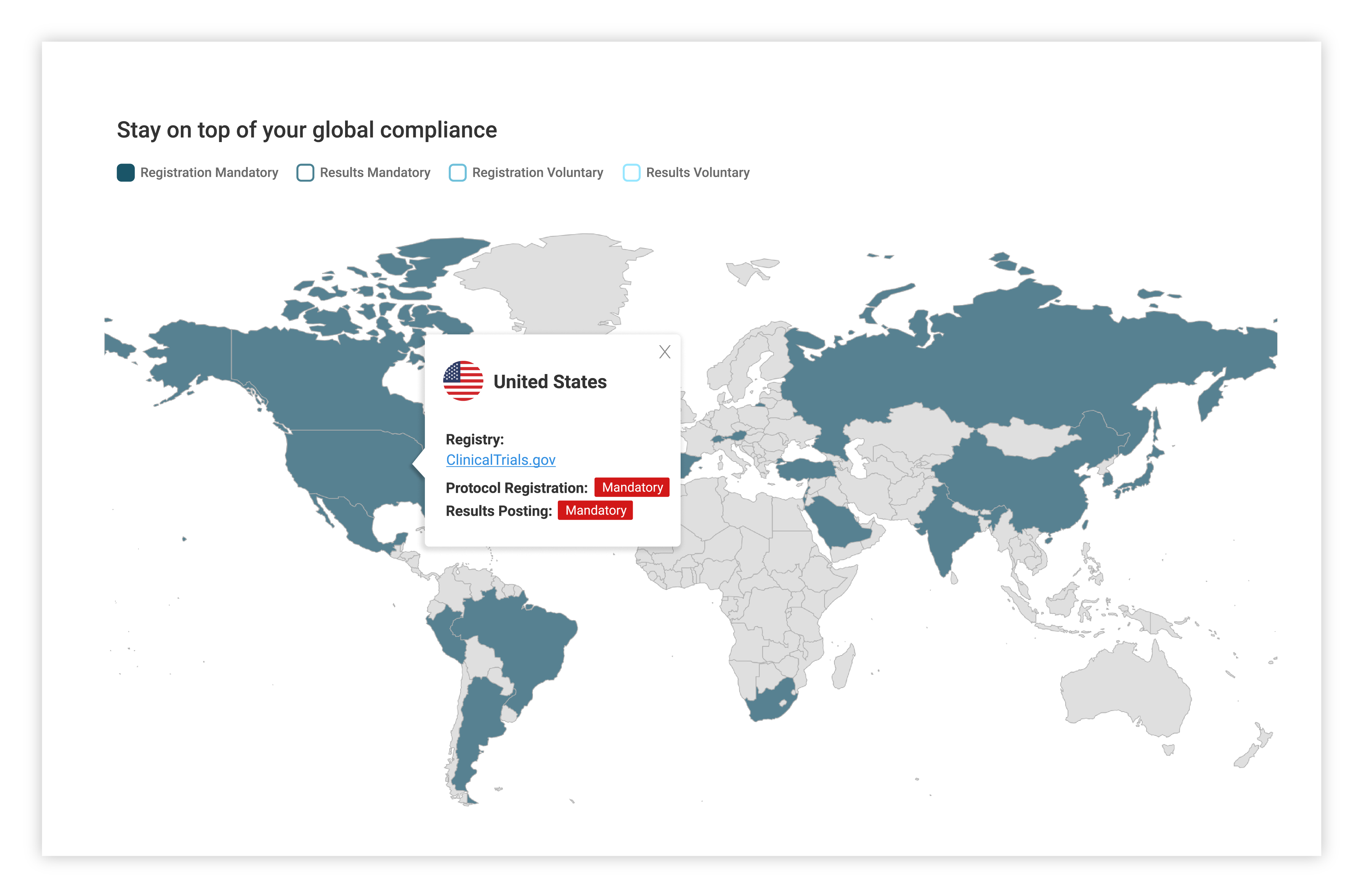
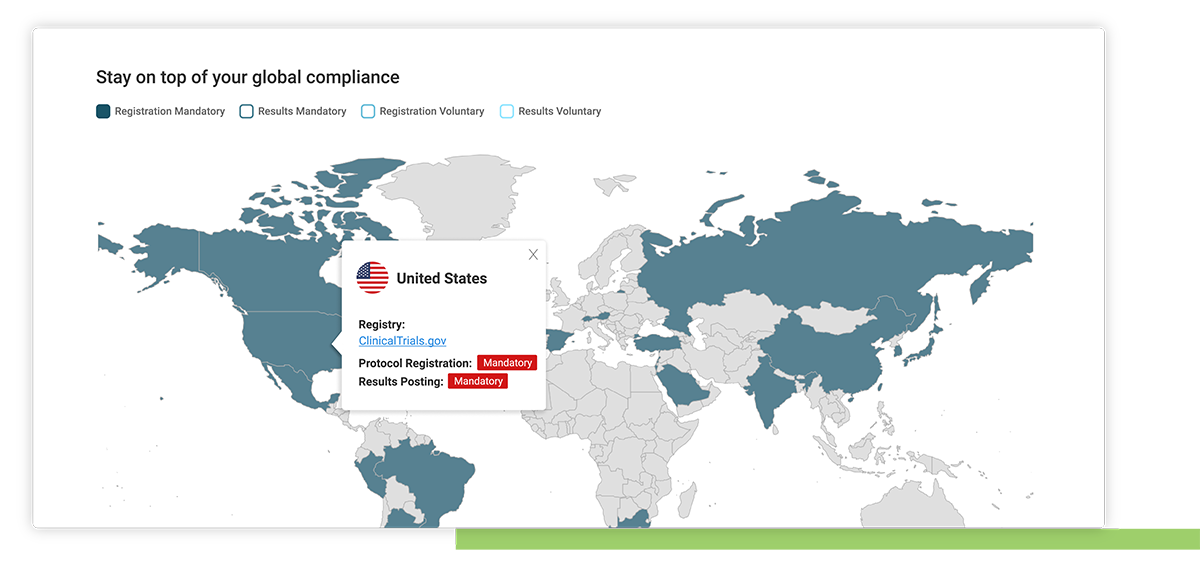
Stay ahead of current regulatory requirements from over 50 countries
Clinical Trial Transparency is constantly evolving with new regulations from around the world. It can be
Country specific regulatory updates
Access to Xogene’s extensive and ongoing research on regulatory updates collated by country.
Country specific regulatory updates
Access to Xogene’s extensive and ongoing research on regulatory updates collated by country.

Country specific regulatory updates
Access to Xogene’s extensive and ongoing research on regulatory updates collated by country.

News feed
Access to daily feed of news articles and press-releases from around the globe, catalogued by date, key words, and country.
News feed
Access to daily feed of news articles and press-releases from around the globe, catalogued by date, key words, and country.

News feed
Access to daily feed of news articles and press-releases from around the globe, catalogued by date, key words, and country.

Monthly Newsletter
Access to Xogene’s monthly analyses of the current and potential regulatory changes and how they impact the transparency requirements.

Monthly Newsletter
Access to Xogene’s monthly analyses of the current and potential regulatory changes and how they impact the transparency requirements.

Monthly digest
Access to Xogene’s monthly analyses of the current and potential regulatory changes and how they impact the transparency requirements.

Got questions?
We're here to help.
Got questions?
We're here to help.
By submitting this form, you agree to Xogene’s Terms of Service and Privacy Policy and also agree to receive emails from Xogene on educational resources, events, and product updates. You can unsubscribe at any time.
About Us
Careers
Solutions
Prime
Reach
RadarX
Services
Regulatory Intelligence
EU CTIS Submission Support
Advisory Services
©2024 Xogene LLC
Terms & Conditions
Privacy Policy
Terms & Conditions